Negative Effects of Endogenous Nitric Oxide on Anti-tumor Photodynamic Therapy
Author: Albert W. Girotti*
Department of Biochemistry, Medical College of Wisconsin, Milwaukee, USA
*Correspondence to: Emeritus Professor. Albert W. Girotti, Department of Biochemistry, Medical College of Wisconsin, Milwaukee, Wi, 53226, USA; E-mail: agirotti@mcw.edu
Received: 27 October 2022; Accepted: 21 November 2022; Published: 28 November 2022
Citation: Girotti AW. (2022) Negative Effects of Endogenous Nitric Oxide on Anti-tumor Photodynamic Therapy, 21st Century Pathology, Volume 2 (6): 133
Abstract
Studies in the author’s laboratory have shown that nitric oxide (NO) produced by many malignant tumors enhances resistance to anti-tumor photodynamic therapy (PDT). Stress-upregulated NO was typically responsible, and it also increased the proliferative and migratory/invasive aggressiveness of PDT-surviving cells as well as non-stressed bystander cells. In this communication, we will briefly review these anti-PDT effects of NO and how they might be mitigated by pharmacological adjuvants.
Keywords:
PDT; NO; Inducible NO synthase; Bystander effects; BET inhibitor
Introduction
Nitric oxide (NO) is a short-lived bioactive free radical molecule that diffuses freely in H2O and tends to localize in hydrophobic milieux such as cellular membranes [1,2]. Many malignant tumors and supporting stroma express inducible nitric oxide synthase (iNOS) which generates nitric oxide (NO) at low levels to activate pro-survival and pro-migration/invasion signaling pathways [3,4]. Endogenous NO can also elicit resistance to anti-tumor radiotherapy or chemotherapy [5]. Several years ago, researchers in the author’s laboratory discovered that this also applies to anti-tumor photodynamic therapy (PDT).
PDT was introduced in the mid-1970s as an innovative new approach for selectively eradicating solid tumors via the generation of cytotoxic reactive oxygen species (ROS). Functional PDT requires (i) a photosensitizing agent (PS), (ii) PS-exciting light, and (iii) molecular oxygen [6]. Most PSs are innocuous until photoactivated, and PDT has few (if any) negative side effects, unlike chemo- or radiotherapy. Specificity is optimized by tumor-localizing PSs and focused far visible-to-near infrared light delivery via fiber-optics. Among several clinically-effective PSs, Photofrin®, was the first to receive FDA approval ~25 years ago [7]. Pro-PSs also exist, the most prominent example being 5-aminolevulinic acid (ALA), which is metabolized to active PS, protoporphyrin IX (PpIX) via the heme biosynthetic pathway, PpIX accumulating initially in mitochondria [8].
Hyper-resistance due to PDT-upregulated iNOS/NO
Many cancers exhibit an innate or acquired resistance to various types of chemotherapy or radiotherapy [9] and it is now clear that resistance mechanisms also exist for PDT. How endogenous NO might affect PDT efficacy was first investigated ca. 22 years ago by two groups using various mouse-borne syngeneic tumors sensitized with Photofrin® [10,11]. It was found that PDT-induced tumor suppression could be much improved when non-specific inhibitors of nitric oxide synthase (NOS) activity (e.g., L-NNA or L-NAME) were present during irradiation. Tumors with the highest constitutive NO output exhibited the greatest sensitivity to NOS inhibition [11]. More recent work with ALA/light-treated tumors produced similar findings [12]. The mechanistic reasoning for all these early findings was that NO-induced vasodilation was counteracting PDT’s anti-tumor vasoconstrictive effects [10-12]. However, at least two questions remained unanswered: (i) whether constitutive/basal NOS/NO is sufficient for hyper-resistance and (ii) which of the three NOS isoforms (1,2) might be most important.
Several years ago, the Girotti AW, et al. (2010) group found that the iNOS isoform (NOS2), was the principal source of NO associated with tumor cell resistance to PDT [13,14]. Here, the term PDT denotes photodynamic “treatment” rather than “therapy”, since these studies were carried out in vitro using cultured human cancer cells (breast, prostate). Importantly, the observed hyper-resistance was mainly due to PDT-upregulated iNOS rather than pre-existing/constitutive enzyme. At the time, such a finding about iNOS/NO was unprecedented for any type of cancer therapy. ALA-induced PpIX was the PS used in these studies [13,14], so it’s important to note that any PpIX exported via the ABCG2 transporter [15] could have also imparted resistance, but in this case not in response to photodynamic stress. Recognition of iNOS/NO involvement in PDT resistance was based on experimental findings such as the following: (i) strong mitigation by iNOS activity inhibitors (1400W, GW274150) or an NO scavenger (cPTIO); (ii) prevention by siRNA-based iNOS knockdown, (iii) “rescuing” iNOS-knockdown cells with GSNO, a chemical NO donor [13,14,16].
In 2017, the above in vitro findings were substantiated at the in vivo level. Immunodeficient (SCID) female mice bearing human breast MDA-MB-231 tumor xenografts were sensitized with ALA-induced PpIX and irradiated with 630 nm LED light. This caused a significant slowdown in tumor growth over a 12-day post-irradiation period. Administration of 1400W or GW274150 during this period slowed growth even further, consistent with iNOS/NO-dependent resistance [17]. Immunoblot and NO analyses on post-PDT tumor samples revealed a progressive increase in iNOS expression and NO output (each reaching 5-6-fold over starting level at 6 h post-PDT). A light-only control was unresponsive to 1400W, indicating that overexpressed, but not basal iNOS/NO, was promoting tumor growth/persistence after a PDT challenge [17]. Consistently, anti-apoptotic Bcl-xL, Survivin, and S100A4 underwent 1400W-inhibitable upregulation after the challenge, whereas pro-apoptotic Bax was down-regulated [17]. Collectively, this was the first known evidence for an anti-tumor therapy being opposed by the iNOS/NO that it can induce.
Hyper-aggressiveness due to PDT upregulated iNOS/NO
In addition to resisting eradication, certain tumor cells that survive PDT stress have been found to exhibit more aggressive behavior than non-treated controls. For example, when human prostate cancer PC3 cells remaining alive (attached) after an ALA/light challenge were continuously monitored beyond 24 hr, a strikingly (~3-fold) increase in proliferation rate was observed relative to dark (ALA-only) controls [18]. This growth spurt was abolished by 1400W or cPTIO, signifying iNOS/NO involvement. Of added significance was the discovery that surviving PC3 cells were more motile, as manifested by increased migration and invasion rates, iNOS/NO again playing a key driving role [18]. Enhanced iNOS/NO-mediated resistance as well as growth and migratory aggressiveness has also been observed for human glioblastoma U87 cells that can withstand PDT stress [19]. For example, in addition to resisting mitochondria-initiated apoptosis, PDT-surviving U87 cells underwent a strong growth and invasiveness spurt which, as with PC3 cells, was 1400W-inhibitable, demonstrating iNOS/NO-dependency [19]. Importantly, this dependency was on stress-upregulated iNOS (>3-fold at 24 hr post-PDT) rather than background iNOS, which was unaffected by 1400W. Matrix metalloproteinases (MMPs) are known to play a key role in cancer cell invasiveness and metastasis. ALA/light stress markedly increased MMP-9 activity of ALA/light-stressed PC3 and U87 cells, inhibition by 1400W, signifying iNOS/NO dependency [19]. Moreover, expression of MMP-9 inhibitor, TIMP-1, was progressively reduced, demonstrating cooperative responses that promoted surviving cell migration/invasion [19]. When obtained, these findings [18,19] were unique in the field of PDT and other oxidative stress-based cancer therapies.
PDT-induced bystander effects
Another example of how iNOS/NO can antagonize PDT was first described in 2017, viz. bystander effects [20]. It was hypothesized that NO from heavily PDT-targeted cells in a tumor would diffuse to non- or poorly-targeted counterparts (bystanders), thereby stimulating growth/migration of the latter. A novel testing approach was devised whereby ALA/light-targeted PC3 cells on a large culture dish were initially separated from non-targeted PC3 bystanders within silicone-rimmed rings. At some point after irradiation, rings were removed, allowing diffusible effectors like NO to diffuse from targeted to bystander cells, which made no physical contact. A striking iNOS upregulation and increase in migration/invasion rate was observed in bystander as well as targeted cells. cPTIO prevented these effects, indicating that NO from targeted cells was the responsible initial driver [20]. This illustrated a NO-mediated ‘feed-forward’ process [21], which extended from the targeted to bystander cell populations. If occurring during clinical PDT, this could promote tumor growth and metastatic expansion.
Conclusion
Exposure of tumor cells to photodynamic stress, e.g., ALA-based PDT, often results in iNOS upregulation with increased resistance to photokilling and accelerated growth, migration, and invasion of surviving cells, NO playing a major role in each of these responses (Figure 1). It is increasingly evident that these responses also occur in oxidative stress-dependent chemo- and radiotherapies [22]. Increased aggressiveness of residual live cells and bystanders, as described for PDT, is a concern that calls for new pharmacologic approaches that can prevent or at least minimize these negative side effects. Recent studies revealed that epigenetic ‘reader’ Brd4 is essential for iNOS transcription in PDT-stressed tumor cells [23]. JQ1, which inhibited Brd4 via BET domain binding, prevented iNOS/NO upregulation by PDT and the elevated cell aggressiveness associated with it [23]. On their own and at very low doses, BET inhibitors like JQ1 are very effective against various malignancies [24]. Thus, there is great promise in using them as PDT adjuvants to limit the described negative effects of iNOS/NO.
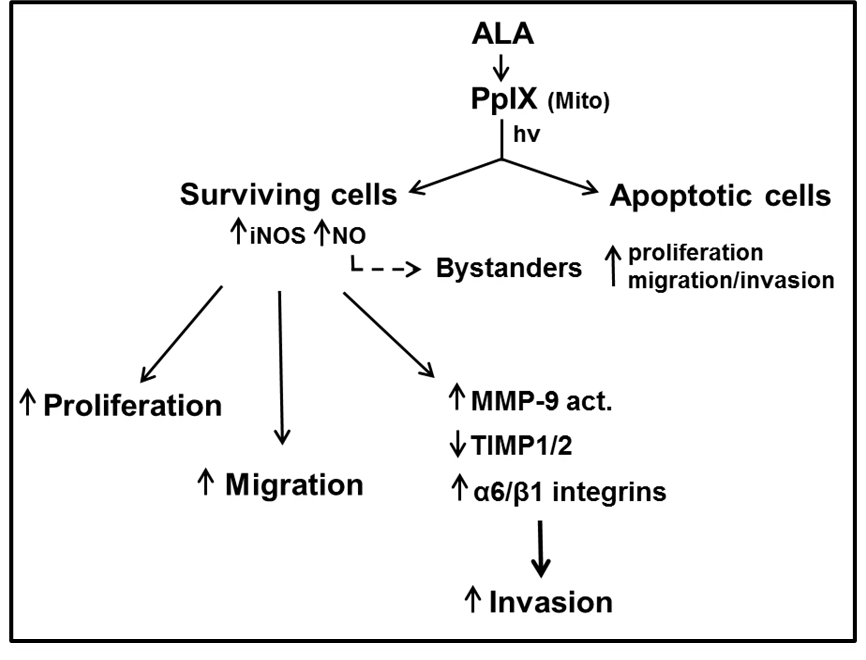
Figure 1: Scheme depicting (a) cancer cell photosensitization by ALA-induced PpIX, (b) PDT challenge with iNOS/NO upregulation, (c) iNOS/NO-stimulated resistance and accelerated proliferation, migration, and invasion of surviving cells and bystanders, (d) supporting changes in key effector proteins.
Conflict of Interest
The authors declare no conflicts of interest.
References
1. Wink DA, Mitchell JB. Chemical biology of nitric oxide: insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radical Biology and Medicine. 1998 Sep 1;25(4-5):434-56. https://doi.org/10.1016/S0891-5849(98)00092-6
2. Ridnour LA, Thomas DD, Donzelli S, Espey MG, Roberts DD, Wink DA, Isenberg JS. The biphasic nature of nitric oxide responses in tumor biology. Antioxidants & redox signaling. 2006 Jul 1;8(7-8):1329-37. https://doi.org/10.1089/ars.2006.8.1329
3. Crowell JA, Steele VE, Sigman CC, Fay JR. Is inducible nitric oxide synthase a target for chemoprevention?. Molecular cancer therapeutics. 2003 Aug;2(8):815-23.
4. Lala PK, Orucevic A. Role of nitric oxide in tumor progression: lessons from experimental tumors. Cancer and Metastasis Reviews. 1998 Mar;17(1):91-106. https://doi.org/10.1023/A:1005960822365
5. Vannini F, Kashfi K, Nath N. The dual role of iNOS in cancer. Redox biology. 2015 Dec 1;6:334-43. https://doi.org/10.1016/j.redox.2015.08.009
6. Agostinis P, Berg K, Cengel KA, Foster TH, Girotti AW, Gollnick SO, Hahn SM, Hamblin MR, Juzeniene A, Kessel D, Korbelik M. Photodynamic therapy of cancer: an update. CA: a cancer journal for clinicians. 2011 Jul;61(4):250-81. https://doi.org/10.3322/caac.20114
7. Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. Photodynamic therapy. JNCI: Journal of the national cancer institute. 1998 Jun 17;90(12):889-905. https://doi.org/10.1093/jnci/90.12.889
8. Peng Q, Berg K, Moan J, Kongshaug M, Nesland JM. 5-Aminolevulinic acid-based photodynamic therapy: principles and experimental research. Photochemistry and photobiology. 1997 Feb 1;65(2):235-51. https://doi.org/10.1111/j.1751-1097.1997.tb08549.x
9. Liu YP, Zheng CC, Huang YN, He ML, Xu WW, Li B. Molecular mechanisms of chemo?and radiotherapy resistance and the potential implications for cancer treatment. MedComm. 2021 Sep;2(3):315-40. https://doi.org/10.1002/mco2.55
10. Henderson BW, Sitnik?Buscr TM, Vaughan LA. Potentiation of photodynamic therapy antitumor activity in mice by nitric oxide synthase inhibition is fluence rate dependent. Photochemistry and photobiology. 1999 Jul;70(1):64-71. https://doi.org/10.1111/j.1751-1097.1999.tb01950.x
11. Korbelik M, Parkins CS, Shibuya H, Cecic I, Stratford MR, Chaplin DJ. Nitric oxide production by tumour tissue: impact on the response to photodynamic therapy. British journal of cancer. 2000 Jun;82(11):1835-43. https://doi.org/10.1054/bjoc.2000.1157
12. Reeves KJ, Reed MW, Brown NJ. The role of nitric oxide in the treatment of tumours with aminolaevulinic acid-induced photodynamic therapy. Journal of Photochemistry and Photobiology B: Biology. 2010 Dec 2;101(3):224-32. https://doi.org/10.1016/j.jphotobiol.2010.07.007
13. Bhowmick R, Girotti AW. Cytoprotective induction of nitric oxide synthase in a cellular model of 5-aminolevulinic acid-based photodynamic therapy. Free Radical Biology and Medicine. 2010 May 15;48(10):1296-301. https://doi.org/10.1016/j.freeradbiomed.2010.01.040
14. Bhowmick R, Girotti AW. Rapid upregulation of cytoprotective nitric oxide in breast tumor cells subjected to a photodynamic therapy?like oxidative challenge. Photochemistry and photobiology. 2011 Mar;87(2):378-86. https://doi.org/10.1111/j.1751-1097.2010.00877.x
15. Palasuberniam P, Yang X, Kraus D, Jones P, Myers KA, Chen B. ABCG2 transporter inhibitor restores the sensitivity of triple negative breast cancer cells to aminolevulinic acid-mediated photodynamic therapy. Scientific reports. 2015 Aug 18;5(1):1-2. https://doi.org/10.1038/srep13298
16. Bhowmick R, Girotti AW. Pro-survival and pro-growth effects of stress-induced nitric oxide in a prostate cancer photodynamic therapy model. Cancer letters. 2014 Feb 1;343(1):115-22. https://doi.org/10.1016/j.canlet.2013.09.025
17. Fahey JM, Girotti AW. Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: Improved outcome with NOS2 inhibitors. Nitric Oxide. 2017 Jan 30;62:52-61. https://doi.org/10.1016/j.niox.2016.12.003
18. Fahey JM, Girotti AW. Accelerated migration and invasion of prostate cancer cells after a photodynamic therapy-like challenge: Role of nitric oxide. Nitric Oxide. 2015 Sep 15;49:47-55. https://doi.org/10.1016/j.niox.2015.05.006
19. Fahey JM, Emmer JV, Korytowski W, Hogg N, Girotti AW. Antagonistic effects of endogenous nitric oxide in a glioblastoma photodynamic therapy model. Photochemistry and photobiology. 2016 Nov;92(6):842-53. https://doi.org/10.1111/php.12636
20. Bazak J, Fahey JM, Wawak K, Korytowski W, Girotti AW. Enhanced aggressiveness of bystander cells in an anti-tumor photodynamic therapy model: Role of nitric oxide produced by targeted cells. Free Radical Biology and Medicine. 2017 Jan 1;102:111-21. https://doi.org/10.1016/j.freeradbiomed.2016.11.034
21. K Hei T, Zhou H, Chai Y, Ponnaiya B, N Ivanov V. Radiation induced non-targeted response: mechanism and potential clinical implications. Current molecular pharmacology. 2011 Jun 1;4(2):96-105. https://doi.org/10.2174/1874467211104020096
22. Girotti AW, Fahey JF, Korytowski W. ROLE of nitric oxide in hyper-aggressiveness of tumor cells that survive various anti-cancer therapies. Critical Reviews in Oncology/Hematology. 2022 Sep 8:103805. https://doi.org/10.1016/j.critrevonc.2022.103805
23. Fahey JM, Stancill JS, Smith BC, Girotti AW. Nitric oxide antagonism to glioblastoma photodynamic therapy and mitigation thereof by BET bromodomain inhibitor JQ1. Journal of Biological Chemistry. 2018 Apr 6;293(14):5345-59. https://doi.org/10.1074/jbc.RA117.000443
24. Filippakopoulos P, Knapp S. Targeting bromodomains: epigenetic readers of lysine acetylation. Nature reviews Drug discovery. 2014 May;13(5):337-56. https://doi.org/10.1038/nrd4286
