Roadmap for Training and Education in Clinical Chemistry and Laboratory Medicine
Authors: Gurmukh Singh1*, Frederick V. Plapp2, Joe Wiencek3
1Department of Pathology, Medical College of Georgia at Augusta University, USA
2Department of Pathology, University of Kansas Medical Center, USA
3Department of Pathology, Vanderbilt University, 1301 Medical Center Drive, USA
*Correspondence to: Professor. Gurmukh Singh, Department of Pathology, Medical College of Georgia, Augusta University, 1120 15th street, Room BI 2008A, Augusta, GA 30912, USA; E-mail: gurmukhsinghmdphd@yahoo.com
Received: 30 November 2022; Revised: 23 December; Accepted: 28 December 2022; Published: 02 January 2023
Copyright: © 2023 Singh G. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Singh G (2023) Roadmap for training and education in Clinical Chemistry and Laboratory Medicine, 21st Century Pathology, Volume 3 (1): 140
Abstract
Background: Training in pathology encompasses a discipline that deals with all the specialties in clinical medicine. Residents in clinical pathology/laboratory medicine are often ill prepared for the role of clinical pathology consultant and medical director of a laboratory.
Content: Based on our collective experience, we have provided a checklist of items to be addressed in clinical chemistry and laboratory medicine education. This list is expected to focus the attention of teachers and trainees on issues essential for gaining competence.
Summary: An organized curriculum for training in clinical chemistry and laboratory medicine is expected to improve quality and efficiency of healthcare.
Keywords:
Clinical Chemistry; Laboratory Medicine; Clinical Pathology; Stewardship of Laboratory
Running title: Laboratory Medicine Training Roadmap
Introduction
Laboratory medicine results drive about 70% of clinical decisions and it is not inappropriate to state, “Without Laboratory Medicine, you are just guessing!” At the same time excessive use of laboratory tests is not only wasteful, but also affects patient safety through unwarranted additional investigations engendered by false positive results. Reference ranges are generally the central 95% of values in a healthy population and, by definition, 5% of the abnormal results would be false positive. The following amusing quote is relevant and cogent, “Remember, ordering a diagnostic test is like picking your nose in public: You must first consider what you will do if you find something” [1].
Clinical chemistry testing accounts for about 60% of the testing activity at a tertiary care hospital and thus constitutes the dominant patient care activity in Clinical Pathology [2]. Most of the clinical chemistry testing is automated and requires little intervention by a pathologist for day-to-day operations. A pathologist’s involvement is essential when a test is modified, new test is introduced, proficiency testing performance issues arise, laboratory renovation, new equipment acquisition, quality management issues, complaints by physician providers, administrative concerns about laboratory utilization and costs, and addressing sentinel events involving laboratory functions. A pathologist/laboratory scientist may generate written laboratory reports requiring interpretation, e.g., protein and hemoglobin electrophoresis results. The pathologist in her/his capacity as the medical director of clinical chemistry/laboratory medicine works closely with the administrative director who generally has responsibility for operational issues. At medical centers with post-graduate training programs, teaching pathology residents is an essential, though under-appreciated, duty of a pathologist/laboratory scientist.
Less than enthusiastic involvement by pathology residents in clinical pathology in general and clinical chemistry, in particular, has been addressed in multiple publications and educational fora at national meetings [2-4]. Part of the “fault” for the tepid interest by pathology residents in clinical chemistry/laboratory medicine rests with us and in our inability to highlight the professional aspects of the discipline. Recent publications have introduced the concept of Entrustable Professional Activities (EPA) and this concept helps to ensure assessing for competency and has a cogent connection to the post-graduate education in pathology [5]. A Graduate Medical Education Committee under the aegis of College of American Pathologists has developed broad goals and recommendations for promoting EPA in pathology [6]. Some sections of pathology have developed similar documents [7,8]. However, a similar approach did not appear to be suitable for the combination of skills, knowledge, and interpersonal interactions in Laboratory Medicine. We have strived to develop a detailed set of issues pertinent for training of residents in clinical chemistry/laboratory medicine. Since clinical chemistry is the dominant subject in Laboratory Medicine, we have chosen to include laboratory administration in our consideration. We have endeavoured to provide a detailed checklist of activities, functions, knowledge, and tools that may be useful in promoting post-graduate education in clinical chemistry and laboratory medicine. We have not included introduction to investigative/research activities, though the discipline provides an excellent opportunity for adding to the medical knowledge through scientific inquiry of the abundant clinical data that flows naturally to clinical pathology.
Billable activities in clinical chemistry are limited essentially to interpretation of protein and hemoglobin electrophoresis results, with the latter often being addressed in hematology section. A potential exists to introduce services for clinical pathology/laboratory medicine consultation as has been done for some functions, most notably, coagulation consultation [9].
The following is submitted to serve as the starting point for promoting training in clinical chemistry and laboratory medicine. The document is purposely formatted as a checklist for ease of implementation and modification to suit the individual interests of an institution. The issues are divided into three major headings, i.e., Laboratory Stewardship, Quality Management, and Interface with clinical care providers and other agencies, though there are overlaps in the functions listed under these headings.
Laboratory Stewardship
The term “Laboratory Stewardship” covers a very wide range of activities, requiring knowledge of laws, regulations, community standards, accreditation requirements, liaison with clinicians, supervision of technical personnel, evaluation of new services, and equipment, laboratory safety, fiduciary responsibility, and disaster management, to name some [10]. These could also be stated as the activities of a laboratory director [11,12].
The essence of laboratory stewardship could be summed up in the following four statements: Provide accurate laboratory results. Provide laboratory results in a timely manner. Ensure execution of the above two functions in a cost-effective manner. Add to the medical knowledge through investigative activities.
Various items of laboratory stewardship are addressed below:
Cost control
For in-patient activities, laboratory is a cost center with no direct revenue other than that included in Part A payments to the hospital. Promoting appropriate utilization of laboratory medicine services includes judicious use of testing.
Management of overuse and inappropriate use of tests
This should be actively managed with the support of the Chief Medical Officer/Vice President for Medical Affairs, and Medical Executive Committee/Board (MEB).
a. Orders for daily tests for N-days: Remove ability to place such orders.
b. Overlapping panels or panel + individual tests: Consolidate overlapping tests, e.g., CMP and bilirubin. Frequency of panel testing should be limited to no more than once a day, outside critical care units.
c. Excessive repeats of monitoring tests, e.g. A1c, Vitamin D. Limit frequency of such order and provide the last result when a test is ordered too early.
d. Limit genetic testing to only once. A repeat should present results of earlier testing and block repeat testing [11].
e. Institute algorithmic testing: e.g., anemia work-up, Celiac disease testing, autoantibody testing, and protein electrophoresis [13].
f. Pros and Cons of laboratory developed panels. Even well-intentioned laboratory developed panels tend to promote overuse.
g. Overuse of reference laboratory tests as discussed below.
h. Judicious use of your authority to deny laboratory tests not meeting the “medical necessity” requirement.
i. Reflex testing management:
i). Cancelling requested tests e.g., serum immunofixation electrophoresis (SIFE) with known and extant monoclonal Ig [14,15].
ii). Adding tests – B12 and prealbumin in patients with low serum folate, SIFE on first discovery of monoclonal Ig [15,16].
Management of reference laboratory tests
Reference Laboratory tests are usually about 1% of the test volume and account for about 10% of the total laboratory expenses. Multiple actions can and should be taken to reduce unwarranted utilization of reference laboratory tests. All requests for reference laboratory tests could be reviewed according to the schema given below:
Dollar value trigger
All tests costing above a certain value (Usually above $200.00) should undergo a review by a healthcare technician and sorted into pools for further examination/review etc. Including pathology residents in such reviews has proven to be an excellent tool for education of residents as well as for promoting appropriate utilization of laboratory testing. Pre-approval for specialists: Some tests/panels could have automatic approval if ordered by an attending of a relevant specialty, e.g., Paraneoplastic syndrome panel ordered by a neurologist. ADAMTS13 testing ordered by a hematologist. Scheduled delay in testing: In case of in-patients, if the results will not alter the treatment, the testing could be shifted to out-patient status. The ethics of this approach could be questioned as the delay would be solely to shift the charges from the hospital to the insurance company or the patient. It often applies to genetic testing. Denials: Tests of limited utility, e.g., Hypercoagulation testing for in-patients, Stool ova and parasites, C diff for patients on laxatives are suitable for denial of service.
All human studies were reviewed and approved by WCG IRB, approval code 1-1410030-1, 5 April 2021.
Revenue enhancement
Laboratory testing in ambulatory setting does generate revenue and there are often paradoxical philosophies about in-patient and ambulatory testing. Laboratory can assist the institution in enhancing revenue by bringing in-house tests being sent to reference laboratories, ensuring proper and timely coding and billing of tests, shifting in-patient tests to ambulatory setting, as medically appropriate. Hospital administration often expects the laboratory to enhance revenue by becoming a “reference laboratory” without realizing the infrastructure needed for such an activity. The laboratory should support institutional activities for outreach, health maintenance testing and providing tests that may reduce costs elsewhere in the institution, e.g., test for impending labor, and test for rupture of membrane.
Diagnostic management teams
A structure for pathologist to provide consultation on difficult diagnostic issue has been espoused by Laposata9. It requires an outstanding expertise in an area that most physicians may not be comfortable with and coagulation testing has been exploited by some pathologists. Molecular testing could provide a similar opportunity. This modality is not in common use due to lack of expertise on our part and reluctance of specialists to cede territory to pathologists.
Equipment acquisition and laboratory renovation/improvement
As the medical/CLIA director, the pathologist is responsible for ensuring sufficient, and safe workspace, adequate staffing, and appropriate equipment for medical needs of the institution. This activity needs close coordination with the administrative director of the laboratories and a long-term view. Attending the annual AACC meeting provides an invaluable opportunity to get oneself acquainted with developments in testing equipment and technologies.
Revenue sources
Payments from government sources, e.g., Medicare and Medicaid are a major source of revenue for the hospitals. The rules and regulations promulgated by the Centers for Medicare and Medicaid Services (CMS) are generally adopted by private insurers as well [17]. The trainees need to acquaint themselves with 3-day and 14-day rules for pre- and post-admission testing, Diagnosis related groups and Relative Value Based Units (RVU): RVUs are the basic component of the Resource-Based Relative Value Scale (RBRVS), which is a methodology used by the Centers for Medicare & Medicaid Services (CMS) and private payers to determine physician payment [18-20].
Part B billing
Very few activities in clinical chemistry qualify for Part B billing, protein electrophoreses sign outs being the main activity. Protein electrophoresis of serum and urine and immunofixation investigation qualify for pathologist sign out and provide an opportunity for Part B billing. (It does not require a pathologist review; however, non-MD clinical scientists are not authorized to bill for the service) [21]. Issues to address are: Gel electrophoresis and Capillary electrophoresis: Pros and cons of the methods. Role of mass-spectrometry in analyzing monoclonal immunoglobulins. Diagnostic criteria for monoclonal gammopathies: Monoclonal gammopathy of undetermined significance, Smoldering multiple myeloma, Multiple/Plasma cell myeloma, Other monoclonal gammopathies. Comments about non-immunoglobulin proteins: Acute and chronic inflammation, Chronic liver disease, Nephrotic syndrome, Elevated transferrin, Low alpha 1 globulin. Reflex testing for SIFE and free light chains. Knowledge of algorithmic testing: Use, overuse, and inappropriate use of tests. Recommendation for additional tests: Underutilization of urine testing. When to communicate directly with a provider: First time detection of a significant monoclonal Ig, especially for tests requested by non-Heme/Onc providers [22]. Clinical Pathology Consultation: Chart review and report to reflect Clinical Pathology consultation. Non-MD sign out and billing: non-MDs are unable to bill for these tests – Ethics of Pathologist countersigning for billing?
Laboratory formulary
Some laboratories have developed and maintained a laboratory formulary, similar to a pharmacy drug formulary. It could serve as a tool in Laboratory Stewardship [23,24].
Quality Management
Laboratory quality can be defined as accuracy, reliability, and timeliness of reported test results. Laboratory quality can be maximized by the implementation of an effective quality management system. Two facets of quality management are quality assurance and quality control.
The trainee should be able to explain the differences between quality management, quality assurance, and quality control and address the following issues: Become familiar with the 12 quality system essentials: organization, customer focus, facilities and safety, personnel, purchasing and inventory, equipment, process management, documents and records, information management, nonconforming event management, assessments, and continual improvement. Participate in the development of QM goals for the coming year. Review standing quality management reports such as: blood culture contamination rate, blood and blood component utilization, critical test result reporting, turn-around time, laboratory test menu changes, proficiency test results, client concerns, safety violations, and corrective actions. Execute at least one quality improvement activity. Critique annual QM reports for the previous year and devise corrective actions for goals not met. Become familiar with quality improvement tools such as: Lean, Lean Six Sigma, Continuous Quality Improvement, Root Cause analysis and Failure Modes Effects Analysis. Attend at least one week of Medical Center Daily Safety Briefing with special consideration of, laboratory safety, specimen integrity, employee injuries, medical waste disposal, and disaster management.
Quality Control and Quality Assurance (QC/QA) Management
Quality assurance is an on-going program for auditing an organization’s processes and systems. Quality control is a measure of precision, or how well the measurement system reproduces the same result over time and under varying operating conditions.
Quality control
a. Understand the role of Westgard rules, and variations on this theme in ensuring laboratory result quality [25,26].
b. Review quality control charts and be able to recognize trends and shifts that occur prior to outright failure.
c. Know how to investigate a QC failure.
d. Know what actions to take after a QC failure.
e. Use of QC materials vs. patient samples. What is commutable material?
f. Understand the advantages and disadvantages of using Moving Averages and Moving Medians to assess quality control.
g. Compare performance of new vs. current reagent lots using patient specimens and QC materials.
h. Compare the results of two instruments using same method.
i. Compare the results of two instruments using different methods.
The three main areas involved in QC failures are: (a) Reagents including quality control materials, calibrators, and analytical reagents; (b) Hardware: This includes the various components of analyzers, e.g., probes, tubing, pumps, light source, cuvettes, power fluctuations, maintenance, and connections. (c) Software: e.g., issues with middleware, connections, upgrades degrading existing protocols, power fluctuations and calculation malfunctions.
In addition to the naturally occurring failures, one should keep the possibility of sabotage at the back of the mind.
QC failure evaluation rules
Laboratories commonly follow Westgard rules for evaluation of QC. QC results of a minimum of twenty control runs, 20 of both high- and low-level controls, on 20 different days are used at the introduction of a new method or new instrument. Mean and standard deviation of the low and high controls are calculated and plotted on a graph paper; Levey-Jennings/Shewhart plot. (New instruments perform these functions electronically and automatically track QC performance.)
For most analytes, once in a 24-hour period, (usually between mid-night and 7 am) on the days on which patient specimens are tested, a minimum of two controls are run and results plotted on the QC graph as shown in the Figure 1. Various types of QC failures are:
A. 12s rule: If one observation exceeds 2 SD from the mean on either the low side or high side, it should serve as a warning of pending issues, but by itself does not constitute QC failure.
B. 13s rule. If either the low or high control is more than 3 SD from the mean, that constitutes failure of QC.
C. 42s rule: If a control is on the same side of the mean on four consecutive days at more than 2SD from the mean, that constitutes QC failure. A variation on this rule is to consider QC failure if two consecutive controls for both low and high level are on the same side of mean at more 2.5 SD.
D. 14s rule. If either control value is more than 4 SD from that of the prior day, or the low and high values on a given day are more than 4 SD apart, that constitutes QC failure.
E. 110s rule. If a control value is on the same side of the mean for 10 consecutive days, even at less than one SD, that constitutes QC failure.
These rules are displayed graphically in the Figure 1.
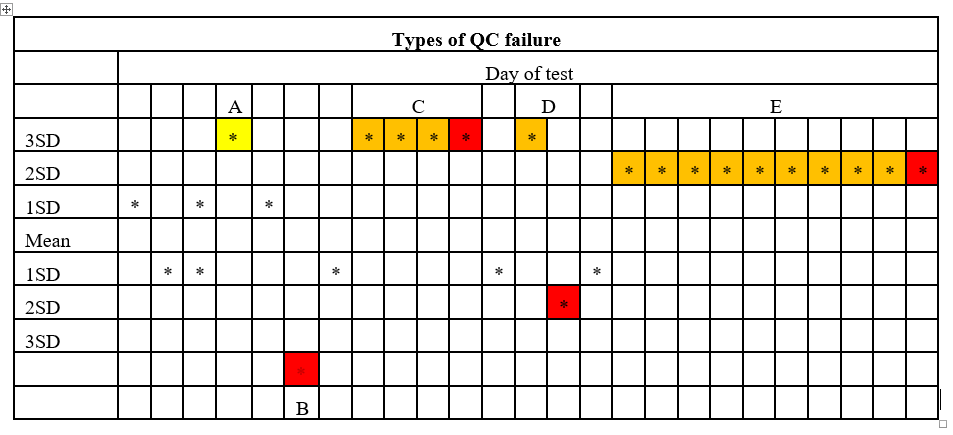
Figure 1: Westgard rules for QC evaluation are graphically presented in the figure. Cells in yellow represent warning but not QC failure. Cells in red represent QC failure and cells in orange represent data preceding outright failure.
Response to QC failure
1. You may repeat the control once, and only once, and if the result is within acceptable limits, that is accepted as adequate. (If you repeat a control multiple times, one of those results is likely to come within acceptable limits, by chance alone.)
2. You may get a fresh lot of controls and repeat the control once. If the control is within normal limits, that would be adequate.
3. If the testing reagents are near their expiration date, you may get a fresh supply of reagents and repeat the QC and if that comes in range, that would be adequate.
4. If the above listed steps do not produce a satisfactory outcome, you may recalibrate the instrument and repeat the QC. If the result is satisfactory, that would be adequate.
5. If the above listed steps fail to resolve the issue, contact the manufacturer of equipment for assistance and repeat the QC after the equipment has been serviced.
It is worth emphasizing that (a) when repeating QC, repeat it only once, (b) do not test any patient specimens till QC issue has been resolved, (c) save all data on a longitudinal basis. Discarding out of range values will narrow the SD beyond workable limits, over time, (d) additional action to be taken after resolving QC failure is presented below.
Failure of QC on one day warrants investigation of time of failure and corrective actions. (Tuesday morning QC failed: Now what do you do?) Once the cause of QC failure has been addressed or using different instrument that is operating within QC parameters, selected specimens from the previous day, at different times, should be retested, e.g., six specimens at least, from each 6-hour period. The results from the analysis should be compared with the results from the prior day to ascertain the time at which QC failed. Additional specimens from the time point after QC failure should be analyzed and the results compared to those from QC adequate analyzer to determine if the results are different in a clinically meaningful way. The amount of variation allowed on repeat testing is available in textbooks26. If the results from the period during which QC failed are not meaningfully different from the QC adequate period, no further action may be needed, other than recording the incident and relevant data.
If the results from the period of QC failure are meaningfully variant, all the specimens from the QC failure period of the prior day should be retested on a QC adequate instrument and corrected results reported to the providers. Providers should also be notified of the error and advised to assess the corrected results.
Proficiency testing evaluation and deficiency response
CLIA-88 requires that laboratories participate in an approved proficiency-testing program for each analyte that is being tested [27]. It is important to address: Understand the difference between an internal quality control program and external proficiency test program. Know how to compare your institution’s proficiency test results to its peer group. Learn how to investigate unacceptable results and document your findings. Understand the implications of failing two out of three PT challenges and three out of three PT challenges. Learn how to analyze the standard deviation index (SDI) to distinguish a random error from bias. Be able to complete a proficiency test corrective action checklist following investigation of a proficiency test failure. How to perform proficiency testing if an external party does not provide materials/programs [28]. Consider going beyond the routine requirements, e.g., investigating if all the results in a challenge are on the same side of mean and four of five results exceed by more than one SDI.
Competency assessment: This issue is applicable to all personnel performing laboratory testing, including physicians and mid-level providers carrying out provider performed microscopy as well as doctoral staff. The timing of competency assessment requires documentation at initial privileging, at six months, and yearly thereafter. The documentation of competency assessment ought to address the 6 elements of competency assessment [29].
Harmonization of Laboratory Tests
A dirty secret of laboratory testing is the variability of test results depending on the test method, with troponin testing being the worst example. International standardization, harmonization, has been accomplished for three analytes, namely, Cholesterol, Creatinine, HbA1c, ± INR.
What did it take to harmonize Creatinine testing and how it affected eGFR? [30].
Harmonization in your institution: Multiple methods for the same analyte on different platforms – Hemoglobin in main lab and Blood Gas instrument, Lactate and Troponin - in main lab and Point of care testing.
New test introduction
Even for FDA approved tests the laboratory needs to address: Verification/validation of a new FDA approved test: Accuracy: Within run precision, Linearity, Carryover, interfering substances. Accuracy: Run a minimum of 20 patient samples on the new method and by a reference method – create a scatter plot; calculate regression (r goal ≥0.95, slope goal 1.0±0.05), Bland Altman bias plot (EP evaluator program) [31]. Carryover: goal < 1.5%. Reference range: Check for validity of vendor or literature reference range against patient samples. Communication to other departments: New specimen requirements; new reference ranges, effect on turn-around time.
Laboratory Developed Test (LDT) Validation
Consult CLSI documents. This issue is under active review by the FDA and regulations are likely to be revised in the near future [32,33]. Spectrum and examples of LDT and laboratory modified tests include: Glucometer use for critically ill patients. FDA approved test for serum/plasma to test other body fluids. Laboratory developed antibody in tissue staining. Laboratory developed assay for early detection of pancreatic, ovarian cancer. Assay for monoclonal light chains [34].
Interface with Clinical Care Providers and Other Agencies
Most of the diagnostic activities occur in the isolation of the laboratory, however, interactions with other healthcare providers, patients and accrediting agencies warrant special consideration. This issue is of particular importance given that pathologists tend to be introverts [35].
Pre-analytical management
It is tempting to limit oneself to the laboratory, however, pre-analytical phase of testing accounts for majority of the so-called “Lab errors” [36,37]. Thus, it behooves us to be involved in the pre-pre-analytical and pre-analytical phase of laboratory testing, through regular communication with users of laboratory services and participation in specimen collection.
Point of Care testing
Point of care tests are designed to be used at or near the site where patients are located, that do not require permanent dedicated space, and that are performed outside the clinical laboratories [38,39]. CLIA 88 categorizes POCT according to complexity or difficulty of performing the test: Waived tests – simple tests with low risk for an incorrect result. Non-waived tests – moderate and high complexity tests. Provider performed tests – microscopy and non-waived tests performed by physicians and midlevel providers in conjunction with the physical examination or treatment of a patient.
Waived Test Regulations
Despite the involvement of FDA and CDC, there are variations in acceptance of waived test, e.g.: The Joint Commission (TJC) recognizes waived test category and expects user to follow manufacturer’s instructions for reagent handling, test performance, maintenance, and quality control. College of American Pathologists (CAP) does not recognize waived tests and applies same regulations as high and moderate complexity tests including CLIA personnel standards, quality control, and proficiency tests. Department of Veterans Affairs (VA) does not recognize waived tests and expects the laboratory director to assume responsibility for all testing in a given institution. Variation among states: Some states, (e.g., Tennessee) do not recognize waived tests.
Non-Waived Test Regulations
All accreditation agencies require compliance with CLIA personnel requirements, training, competency, calibration, quality control, and proficiency testing.
Provider Performed Tests: CAP requires training, competency assessment, and quality management system including instrument maintenance, reagent storage, quality control and proficiency testing.
Critical Values
Initially called panic values, some abnormal values warrant communication of the results to a responsible provider and require an institution wide approach to ensure proper communications [40-42]. The trainees should become acquainted with: How to develop roster of critical values. Reporting methods: Phone, Secure messaging, closing the loop, e-methods [42-45]. Performance measures for critical value reporting. Ways to reduce the workload of critical value reporting [46,47]. Delta check. Causes of variations in values. Consider “Hypercritical Values” [41]. Consider using secure messaging for reporting critical values [42].
Laboratories have different policies regarding repeating critical lab result prior to reporting. A Q-Probes study by the College of American Pathologists reported that 61% of clinical laboratories delay communication of critical values by as much as 20 minutes because they repeat the test to confirm the result before reporting [45,46]. More than 99% of initial and repeat critical results were deemed to not be significantly different46. These results confirmed the findings of a previous study which showed that repeat testing of critical hemoglobin, platelet count, WBC count, prothrombin time and activated partial thromboplastin time results did not offer any advantage over singlet testing. Even though critical values occur at the upper and lower ends of the analytical range, these results are just as reliable as normal results in a well-managed laboratory. Repeat testing may do more harm than good by delaying reporting of critical results [48,49].
Stat test management
This is essentially a legacy term from the days when laboratory tests were not as automated and turnaround time was in hours. By judicious use of point of care testing and automation, the turn-around time of tests that could justifiably be ordered stat can be reduced to less than 45 minutes, thus obviating the need for stat tests [39,43].
Liaisons with Clinical Colleagues
The importance of informal contacts with clinical colleagues cannot be over-emphasized. These may be ad-hoc encounters in cafeteria, hallways, or at scheduled activities such as tumor boards, medical staff meetings, contacts regarding laboratory issues, teaching activities and social interactions. A formal channel for communication with clinical colleagues is through the Medical Executive Board (MEB). The following are some of the triggers for contact with MEB.
Presentation to Medical Executive Board (MEB) for approval of change in service. Usually this is accomplished by notifying Chief Medical Officer (CMO). On rare occasions, the medical director may need to make a presentation to the MEB when multiple departments may be affected, e.g., change in method/instrument for intra-operative monitoring of heparin [43].
Adding new tests/modifying existing test. New tests may be added due to change in medical science, e.g., introduction of assay for serum free light chains, Cystatin C assay to provide race neutral eGFR, Point of Care molecular testing for SARS CoV-2; adding tests at the request of a clinical department to support new service, e.g., introduction of in-house testing for Cortisol and Aldosterone to support Adrenal Center performing venous samples from adrenal veins.
Removing old tests from menu. MEB notification is prudent before removing tests judged to be non-value added, e.g., Red Cell folate, placental microglobulin testing in vaginal fluid for detection of rupture of membranes, cessation of viral cultures in favor of nucleic acid testing.
Reflex testing. The Laboratory Policy on reflex testing should be updated, at least yearly, and submitted to MEB through the CMO.
Critical value menu changes. Changes in critical values may be requested by a clinical department mostly to reduce the number of false alarms, or due to change in testing methodology, e.g., high sensitivity troponin assay.
Selection or change in reference laboratory. While it is the prerogative of the CLIA lab director to select one or more laboratories for tests sent to outside laboratories, it is imperative to seek MEB approval of the selection. Laboratory Formulary may be an aid in this endeavour [23,24].
Accreditation Inspection of Laboratories
Centers for Medicare and Medicaid Services has delegated this function to outside entities termed deemed entities. College American Pathologists (CAP) and The Joint Commission (TJC) are the major deemed entities. States have the authority to conduct their own inspections.
While residents usually participate in Self-inspection, inspection of outside laboratory is an invaluable experience in preparation to be laboratory director and the rules and be knowledgeable about regulations pertinent to accreditation of the laboratory, as required by CMS. The residents would need to take the laboratory inspector course and participate in peer review of laboratory and learn the rule of the game, such as on-site correction, consultation with CAP office during an accreditation inspection. Inspecting an outside laboratory is always an educational experience about how to do somethings better and things not to do.
Note: College of American Pathologists kindly supports the participation by residents in on-site inspection both as inspectors, but more importantly as trainees in addition to the usual complement of recommend number of inspectors.
Substances of abuse testing
Such testing may be part of clinical care and does not require additional consideration. However, if the testing has medico-legal implications, e.g., employee screening, pre-employment testing, or newborn testing for drug abuse by the mother, then additional measures need to be ensured [50]. e.g.: Witnessed specimen collection. Screening and confirmation: Screening and confirmation should employ different technologies. Screening is usually done by immunoassays and confirmation by Mass-Spectrometry50. Forensic testing with chain of custody. Implications of meconium testing for substances of abuse. Forensic testing may require court appearance.
Soft Skills
How to influence people who do not report to you, but you are responsible for what they do? Liaison with Laboratory Personnel. Treat with respect. Take care of the people who take care of you. The issues usually requiring attention are: Liaison with Administrative Laboratory Director. Familiarity with techniques for conflict resolution. Providing constructive feedback. Evaluations: Performance evaluation of doctoral staff. Guidance on time management. Interaction with Clinical Colleagues: Paradoxes – (i) Supporting patient care and (ii) Stewardship of Laboratory.
Practice for job interview: Go through a dress rehearsal for a job interview, record the session and view your performance. Seek input from colleagues and attendings [51].
Disaster Management Planning and Operation
Preparation and maintenance of skills for events that may never happen takes positive effort and refresher training especially for the following facets: Define essential test menu that must be performed in event of mass absenteeism. Maintain an off-site laboratory that can meet essential test needs. Develop a reciprocal arrangement with a nearby laboratory to provide support during a disaster. Retain laboratory records on duplicate servers. Maintain a list of available point of care instruments to supplement testing on or off-site. Have a contingency plan for an accessory morgue in the event of increased fatalities. Know how to undertake root cause analysis and Failure Modes Effects Analysis. Become familiar with the WHO’s “The selection and use of essential in vitro diagnostics” [52]. Become familiar with “CLSI GP36-A: Planning for Laboratory Operations During a Disaster; Approved Guideline”.
Ethics in clinical practice and teaching
You made a mistake – now what?
How to deal with “Very Important Person” [53].
Pro-bono testing for hospital staff and family members.
Maintaining safe, harassment free, nurturing environment.
Stark Law and other laws relevant to laboratory [54]. Malpractice and legal issue [10].
Community Service
Participating in health maintenance activities of the community.
Answering questions about health in general and Laboratory Medicine, in particular, from lay public, at Health Tap.
Concluding Remarks
The residents in pathology enter the training programs with multiple goals. From a pragmatic and resident centered point of view the important goals are:
Pass the pathology boards on first attempt.
Get into the fellowship program of one’s choice.
Find a meaningful job on completion of training.
The broader goals are:
Be knowledgeable enough to be a consultant to clinical colleagues regarding Laboratory Medicine.
Gain the knowledge, skills, and competence to function as a laboratory medical director.
Engage in activities that improve healthcare and add to the medical knowledge.
While all these goals are interlinked the main thrust of this communication is to prepare pathology residents to be medical directors of laboratories.
Conflict of Interest
None declared.
References
1. Paes BA, Modi A, Dunmore R. Changing physicians' behavior using combined strategies and an evidence-based protocol. Archives of Pediatrics & Adolescent Medicine. 1994 Dec 1;148(12):1277-80. https://doi.org/10.1001/archpedi.1994.02170120039006
2. Singh G, Bollag RJ, Savage NM. Engaging pathology residents in clinical chemistry: the essential ingredient is a committed teacher. The Journal of Applied Laboratory Medicine. 2021 Mar;6(2):522-31. https://doi.org/10.1093/jalm/jfaa140
3. Haidari M, Yared M, Olano JP, Alexander CB, Powell SZ. Attitudes and beliefs of pathology residents regarding the subspecialty of clinical chemistry: results of a survey. Archives of Pathology & Laboratory Medicine. 2017 Feb;141(2):203-8. https://doi.org/10.5858/arpa.2015-0547-OA
4. Wiencek J, Blick K, Laposata M. Making the quantum leap in clinical chemistry teaching. 2019 Annual AACC meeting scientific session, 33217, August 6, 2019.
5. Ten Cate, O. Nuts and bolts of entrustable professional activities. Journal of Graduate Medical Education. 2013;5(1):157-8. https://doi.org/10.4300/JGME-D-12-00380.1
6. McCloskey CB, Domen RE, Conran RM, Hoffman RD, Post MD, Brissette MD, Gratzinger DA, Raciti PM, Cohen DA, Roberts CA, Rojiani AM. Entrustable professional activities for pathology: recommendations from the College of American Pathologists Graduate Medical Education Committee. Academic Pathology. 2017 Jul 17;4:2374289517714283. https://doi.org/10.1177/2374289517714283
7. Cotta CV, Ondrejka SL, Nakashima MO, Theil KS. Pathology Residents as Testing Personnel in the Hematology LaboratoryDeveloping Entrustable Professional Activities. Archives of Pathology & Laboratory Medicine. 2022 Jul 1;146(7):894-902. https://doi.org/10.5858/arpa.2020-0630-OA
8. Pagano MB, Treml A, Stephens LD, Joshi S, Li Y, Lopez?Plaza I, Poyyapakkam S, Schwartz J, Tanhehco Y, Zantek ND. Entrustable professional activities for apheresis medicine education. Transfusion. 2020 Oct;60(10):2432-40. https://doi.org/10.1111/trf.15983
9. Verna R, Velazquez AB, Laposata M. Reducing diagnostic errors worldwide through diagnostic management teams. Annals of Laboratory Medicine. 2019 Mar 1;39(2):121-4. https://doi.org/10.3343/alm.2019.39.2.121
10. Peterson Jr JE. Survey of medical malpractice cases and settlements with pathology or laboratory standard of care issues. Seminars in Diagnostic Pathology. 2019 Sep 1 (Vol. 36, No. 5, pp. 366-371). WB Saunders. https://doi.org/10.1053/j.semdp.2019.06.006
11. Laboratory Director – Regulatory requirements: 42 CFR § 493.1445 - Standard; Laboratory director responsibilities.
12. Clinical Laboratory Improvement Amendments (CLIA). What are my responsibilities as a Laboratory Director? https://www.cms.gov/regulations-and-guidance/legislation/clia/downloads/brochure7.pdf
13. Miller CE, Krautscheid P, Baldwin EE, Tvrdik T, Openshaw AS, Hart K, LaGrave D. Genetic counselor review of genetic test orders in a reference laboratory reduces unnecessary testing. American Journal of Medical Genetics Part A. 2014 May;164(5):1094-101. https://doi.org/10.1002/ajmg.a.36453
14. Heaton C, Vyas SG, Singh G. Audit of use and overuse of serum protein immunofixation electrophoresis and serum free light chain assay in tertiary health care: a case for algorithmic testing to optimize laboratory utilization. American Journal of Clinical Pathology. 2016 Apr 1;145(4):531-7. https://doi.org/10.1093/ajcp/aqw026
15. Zia HM, Singh G. Optimization of utilization of serum protein analysis: role of the electronic medical record in promoting consultation by pathology. American Journal of Clinical Pathology. 2013 Jun 1;139(6):793-7. https://doi.org/10.1309/AJCP1ZRZ7KLYSLTG
16. Kozman D, Mattox S, Singh G. Serum folate of less than 7.0 ng/mL is a marker of malnutrition. Laboratory Medicine. 2020 Sep 1;51(5):507-11. https://doi.org/10.1093/labmed/lmz101
17. Centers for Medicare and Medicaid Services Payment System: Part A, Part B, Part C and Part D. (https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c16.pdf)
18. Three Day Payment Window. Centers for Medicare & Medicaid Services.
https://hh-law.com/insights/articles/cms-publishes-revision-to-laboratory-14-day-rule/
19. Diagnosis related groups (DRG) https://www.cms.gov/Medicare/Medicare-Fee-for-Service
20. Practice Management. What Are Relative Value Units (RVUs)? AAPC. https://www.aapc.com/practice-management/rvus.aspx
21. Medicare Claims Processing Manual Chapter 16 - Laboratory Services. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c16.pdf
22. Singh G. Serum and urine protein electrophoresis and serum-free light chain assays in the diagnosis and monitoring of monoclonal gammopathies. The Journal of Applied Laboratory Medicine. 2020 Nov;5(6):1358-71. https://doi.org/10.1093/jalm/jfaa153
23. Jackson BR. Laboratory formularies. Clinica Chimica Acta. 2014 Jan 1;427:151-3. https://doi.org/10.1016/j.cca.2013.09.040
24. Zhang YV, Smoller BR, Levy PC. Laboratory formulary: a model for high-value evidence-based medicine. Clinical Chemistry. 2017 Jul 1;63(7):1299-300. https://doi.org/10.1373/clinchem.2016.270819
25. Poh DK, Lim CY, Tan RZ, Markus C, Loh TP. Internal quality control: Moving average algorithms outperform Westgard rules. Clinical Biochemistry. 2021 Dec 1;98:63-9. https://doi.org/10.1016/j.clinbiochem.2021.09.007
26. Miller GW. In Henry’s Clinical diagnosis and management by laboratory methods. 2022. Chapter 11. Edition 24, McPherson RA, Pincus MR eds. Elsevier Saint Louis, Missouri, IBSN: 978-0-323-67320-4.
27. Clinical Laboratory Improvement Amendments (CLIA). Centers for Disease Control and Prevention.
https://www.cdc.gov/clia/index.html
28. Agarwal A, Gupta S, Sharma RK, Finelli R, Kuroda S, Vij SC, Boitrelle F, Kavoussi P, Rambhatla A, Saleh R, Chung E. Post-Vasectomy Semen Analysis: Optimizing Laboratory Procedures and Test Interpretation through a Clinical Audit and Global Survey of Practices. The World Journal of Men's Health. 2022 Jul;40(3):425-41. https://doi.org/10.5534/wjmh.210191
29. What Do I Need to Do to Assess Personnel Competency? Centers for Medicare & Medicaid Service. https://www.cms.gov/regulations-and-guidance/legislation/clia/downloads/clia_compbrochure_508.pdf
30. Greg Miller W, Myers GL, Ashwood ER, Killeen AA, Wang E, Thienpont LM, Siekmann L. Creatinine measurement: state of the art in accuracy and interlaboratory harmonization. Archives of Pathology & Laboratory Medicine. 2005 Mar;129(3):297-304. https://doi.org/10.5858/2005-129-297-CMSOTA
31. EP Evaluator. Data Innovations. https://www.datainnovations.com/ep-evaluator-0
32. Genzen JR, Mohlman JS, Lynch JL, Squires MW, Weiss RL. Laboratory-developed tests: a legislative and regulatory review. Clinical Chemistry. 2017 Oct 1;63(10):1575-84. https://doi.org/10.1373/clinchem.2017.275164
33. Laboratory Developed Tests. US Food & Drug Adminstratoin. https://www.fda.gov/medical-devices/in-vitro-diagnostics/laboratory-developed-tests
34. Wilhite D, Arfa A, Cotter T, Savage NM, Bollag RJ, Singh G. Multiple myeloma: Detection of free monoclonal light chains by modified immunofixation electrophoresis with antisera against free light chains. Practical Laboratory Medicine. 2021 Nov 1;27:e00256. https://doi.org/10.1016/j.plabm.2021.e00256
35. Jafrani S, Zehra N, Zehra M, Ali SM, Mohsin SA, Azhar R. Assessment of personality type and medical specialty choice among medical students from Karachi; using Myers-Briggs Type Indicator (MBTI) tool. JPMA. 2017 Apr;67(4):520-526.
36. Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clinical Chemistry and Laboratory Medicine (CCLM). 2006 Jun 1;44(6):750-9. https://doi.org/10.1515/CCLM.2006.123
37. Lima-Oliveira G, Volanski W, Lippi G, Picheth G, Guidi GC. Pre-analytical phase management: a review of the procedures from patient preparation to laboratory analysis. Scandinavian Journal of Clinical and Laboratory Investigation. 2017 Apr 3;77(3):153-63. https://doi.org/10.1080/00365513.2017.1295317
38. Florkowski C, Don-Wauchope A, Gimenez N, Rodriguez-Capote K, Wils J, Zemlin A. Point-of-care testing (POCT) and evidence-based laboratory medicine (EBLM)–does it leverage any advantage in clinical decision making? Critical Reviews in Clinical Laboratory Sciences. 2017 Nov 17;54(7-8):471-94. https://doi.org/10.1080/10408363.2017.1399336
39. Singh G, Savage NM, Gunsolus B, Foss KA. Requiem for the STAT test: automation and point of care testing. Laboratory Medicine. 2020 Mar 10;51(2):e27-31. https://doi.org/10.1093/labmed/lmz080
40. Lundberg GD. Critical (panic) value notification: an established laboratory practice policy (parameter). Jama. 1990 Feb 2;263(5):709. https://doi.org/10.1001/jama.1990.03440050103044
41. Newitt VN. Labs add safety net to critical values procedure. CAP today, March 2019. https://www.captodayonline.com/labs-add-safety-net-to-critical-values-procedure/
42. Clavijo A, Fallaw D, Coule P, Singh G. Communication of critical laboratory values: optimization of the process through secure messaging. Laboratory Medicine. 2020 Jan 2;51(1):e6-11. https://doi.org/10.1093/labmed/lmz047
43. Thompson TZ, Kunak RL, Savage NM, Agarwal S, Chazelle J, Singh G. Intraoperative monitoring of heparin: Comparison of activated coagulation time and whole blood heparin measurements by different point-of-care devices with heparin concentration by laboratory-performed plasma anti-Xa assay. Laboratory Medicine. 2019 Oct 10;50(4):348-56. https://doi.org/10.1093/labmed/lmz014
44. Barenfanger J, Sautter RL, Lang DL, Collins SM, Hacek DM, Peterson LR. Improving patient safety by repeating (read-back) telephone reports of critical information. American Journal of Clinical Pathology. 2004 Jun 1;121(6):801-3. https://doi.org/10.1309/9DYM6R0TM830U95Q
45. Howanitz PJ, Steindel SJ, Heard NV. Laboratory critical values policies and procedures: a college of American Pathologists Q-Probes Study in 623 institutions. Archives of Pathology & Laboratory Medicine. 2002 Jun;126(6):663-9. https://doi.org/10.5858/2002-126-0663-LCVPAP
46. Toll AD, Liu JM, Gulati G, Behling EM, Kocher WD. Does routine repeat testing of critical values offer any advantage over single testing?. Archives of Pathology & Laboratory Medicine. 2011 Apr;135(4):440-4. https://doi.org/10.5858/2010-0025-OA.1
47. Lehman CM, Howanitz PJ, Souers R, Karcher DS. Utility of repeat testing of critical values: a Q-probes analysis of 86 clinical laboratories. Archives of Pathology and Laboratory Medicine. 2014 Jun;138(6):788-93. https://doi.org/10.5858/arpa.2013-0140-CP
48. Emancipator K. Critical values: ASCP practice parameter. American Journal of Clinical Pathology. 1997 Sep 1;108(3):247-53. https://doi.org/10.1093/ajcp/108.3.247
49. Genzen JR, Tormey CA. Pathology consultation on reporting of critical values. American Journal of Clinical Pathology. 2011 Apr 1;135(4):505-13. https://doi.org/10.1309/AJCP9IZT7BMBCJRS
50. Van Wijk XM, Goodnough R, Colby JM. Mass spectrometry in emergency toxicology: Current state and future applications. Critical Reviews in Clinical Laboratory Sciences. 2019 May 19;56(4):225-38. https://doi.org/10.1080/10408363.2019.1585415
51. Singh G, Savage NM, Bollag RJ, Booker D. Pathology Job Search and Interview: Perspectives of the United States Experience. Archives of Pathology and Laboratory Medicine. In press.
52. Schroeder LF, Guarner J, Amukele TK. Essential diagnostics for the use of World Health Organization essential medicines. Clinical Chemistry. 2018 Aug 1;64(8):1148-57. https://doi.org/10.1373/clinchem.2017.275339
53. Nurok M, Gewertz B. The High-Profile Patient—Ensuring Good Care for the Entire Hospital. JAMA Surgery. 2019 Feb 1;154(2):105-6. https://doi.org/10.1001/jamasurg.2018.3537
54. A Roadmap for New Physicians Fraud & Abuse Laws. U.S. Department of Health and Human Services Office of Inspector General. https://oig.hhs.gov/compliance/physician-education/fraud-abuse-laws
